






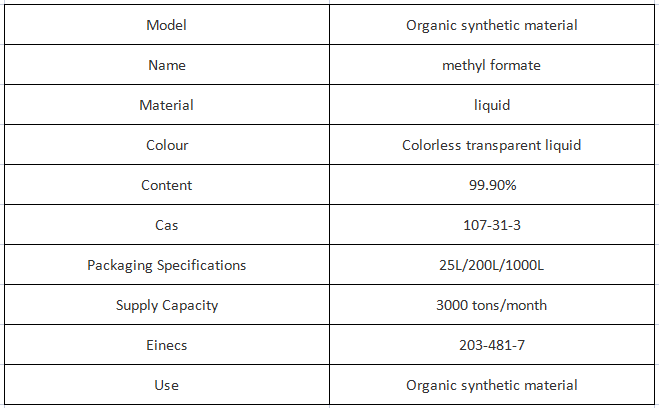



Methyl formate, a clear, volatile liquid, is the simplest carboxylate ester. It can be made in the lab by the acid-catalyzed esterification of formic acid and methanol. Industrially, it is produced by the base-catalyzed reaction of methanol and carbon monoxide.
The industrial uses of methyl formate include the manufacture of other formic acid derivatives, as a blowing agent for foams, and as an agricultural fumigant. It was formerly used as a refrigerant as an alternative to sulfur dioxide even though it also poses a toxicity hazard.
